Hello everyone, In this Article we will explore the Carbohydrates and develop the understanding about its various types, function, Importance, and it’s molecular structure.

- Carbohydrates are always to be well-known macromolecules, particularly in relation to our dietary choices.
- Nowadays “Low-carb” diets are more popular for weight loss however the athletes often “carb-load” before competitions for energy.
- Carbohydrates are essential parts of our diet and are available in ample amounts in grains, fruits, and vegetables.
- They provide energy to the body, primarily through glucose, a simple sugar found in starch and staple foods.
- The carbohydrates can be represented in stoichiometric formula (CH2O)n in which the ratio of carbon to hydrogen to oxygen is 1:2:1.
- It plays a vital role in all living beings like humans, animals, and plants.
- The term “carbohydrate” has been derived from the composition of the components of water and carbon.
- Carbohydrates are broadly classified into three subcategories like monosaccharides, disaccharides, and polysaccharides.
Prepare to delve into the captivating world of carbohydrates, where complexity and intrigue intertwine. Represented by the stoichiometric formula (CH2O)n, where n denotes the carbon count, carbohydrates reveal a remarkable ratio of carbon to hydrogen to oxygen : 1:2:1. This formula sheds light on the term “carbohydrate” itself, stemming from the fusion of carbon (“carbo”) and the constituents of water (“hydrate”). Within this realm, carbohydrates take shape in three distinctive subtypes: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
Monosaccharides (mono means “one”; sacchar means “sweet”) are simple sugars, which are Glucose, Fructose, Galactose. Among all the most common is glucose. Carbohydrates are a well-known macromolecule, particularly in relation to our dietary choices. Glucose, a familiar friend, reigns as the most prevalent monosaccharide. Their carbon count typically spans from three to seven, with names culminating in the suffix “-ose.” An aldehyde group in the sugar structure earns it the title of an aldose, while a ketone group designates a ketose. Trioses (three carbons), pentoses (five carbons), and hexoses (six carbons) emerge as monosaccharide classifications, each holding its own allure. Visualize the monosaccharides in Figure 1, an illustrative guide to their captivating nature.

How does the Energy Production from Carbohydrates (Cellular Respiration ) ?
Any monosaccharide (simple sugar) can be converted into energy that the cell can utilise. In plants and animals, extra carbohydrates are stored as starch and glycogen, respectively, and are ready to be metabolised in the event that the organism’s energy requirements unexpectedly rise.
Carbohydrates are broken down into monosaccharides, which are subsequently distributed to all the live cells of an organism when those energy demands rise. One such example of a monosaccharide utilised for energy production is glucose (C6H12O6).
Each sugar molecule undergoes a protracted series of chemical processes inside the cell in order to be broken down. High-energy adenosine triphosphate (ATP) molecules are created as chemical energy is liberated from the monosaccharide’s bonds. All cells use ATP as their principal form of energy. same as the bone is used as currency to buy goods, cells use motes of ATP to perform immediate work and power chemical responses.
The breakdown of glucose during metabolism is call cellular respiration can be described by the equation
C6H12O6 + 6O2 → 6H2O + 6CO2 + energy
How does the Plants Producing Carbohydrates (Photosynthesis) ?
Plants and certain organisms produce carbohydrates, such as glucose, through a process called photosynthesis. This process involves converting light energy into chemical energy by combining carbon dioxide gas molecules (CO2) to form sugar molecules. It is important to note that photosynthesis requires energy input in the form of light to facilitate the synthesis of glucose.
6H2O + 6CO2 + energy → C6H12O6 + 6O2
Glucose molecules undergo chemical processes in plants, where they can be combined with and transformed into different types of sugars. Within plants, glucose is stored as starch, which can be broken down through cellular respiration to produce glucose again, thus providing ATP.
Glucose has the chemical formula C6H12O6. Glucose is a significant source of energy for people. Energy from glucose is released during cellular respiration, and this energy is used to produce adenosine triphosphate (ATP). Using carbon dioxide and water, plants produce glucose, which is then utilised for the plant’s energy needs. Additional glucose is frequently stored as starch, which is then broken down by cells in humans and other animals that consume plants.
Other typical monosaccharides include galactose and fructose, which are both present in milk sugars and fruit sugars, respectively. All three of these monosaccharides—glucose, galactose, and fructose—have the same chemical formula (C6H12O6), but because of the different configurations of functional groups surrounding the asymmetric carbon, they differ structurally and chemically (and are referred to as isomers) (Figure 2).
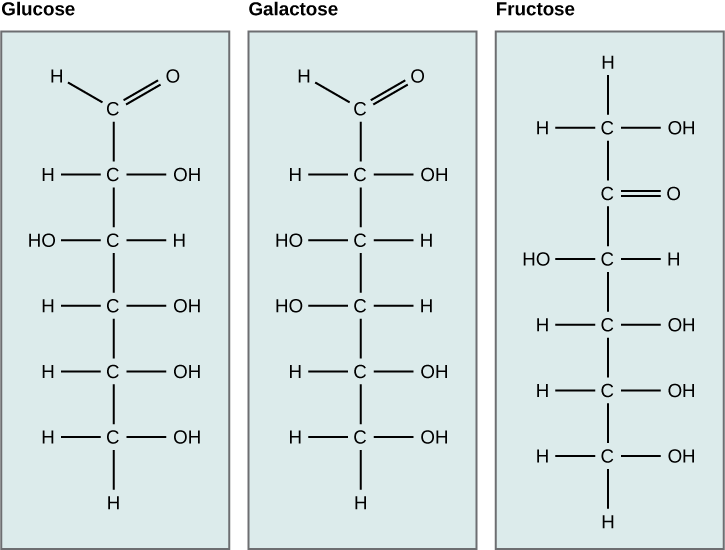
What kind of sugars are these, aldose or ketose?
Monosaccharides can exist in two forms: as a linear chain or as ring-shaped molecules. In aqueous solutions, they are typically found in ring forms. Glucose, for example, can exist in a ring form with two different arrangements of the hydroxyl group (-OH) around the anomeric carbon (carbon 1). When the hydroxyl group is located below carbon number 1, it is referred to as the alpha (α) position. Conversely, when it is above the plane, it is referred to as the beta (β) position.

Disaccharides
Disaccharides (di means “two”) are formed when two monosaccharides undergo a dehydration reaction, also known as a condensation reaction or dehydration synthesis. In this process, the hydroxyl group of one monosaccharide combines with the hydrogen of another monosaccharide, resulting in the release of a water molecule and the formation of a covalent bond. This covalent bond, which occurs between a carbohydrate molecule and another molecule (in this case, between two monosaccharides), is called a glycosidic bond. Glycosidic bonds, also referred to as glycosidic linkages, can exist in two forms: alpha or beta. An alpha bond is formed when the OH group on the carbon-1 of the first glucose is positioned below the ring plane, while a beta bond is formed when the OH group on the carbon-1 is positioned above the ring plane.

Common disaccharides include lactose and maltose(Figure 5). Lactose is made up of glucose and galactose monomers and is naturally found in milk. Maltose, also known as malt sugar, is formed when two glucose molecules undergo a dehydration reaction. Among the common disaccharides, sucrose, also referred to as table sugar, is the most prevalent. It consists of glucose and fructose monomers.

Polysaccharides
Polysaccharides(Poly means many) are long chains of monosaccharides linked together by glycosidic bonds. These chains can be branched or unbranched, and they can consist of different types of monosaccharides. The molecular weight of a polysaccharide can be 100,000 daltons or more, depending on the number of monomers joined. Starch, glycogen, cellulose, and chitin are some primary examples of polysaccharides.
Starch, which is composed of amylose and amylopectin, serves as the stored form of sugars in plants. When plants produce glucose in excess of their immediate energy requirements, it is stored as starch in various plant parts like roots and seeds. In seeds, the starch serves as a source of food for the embryo during germination and can also be consumed by humans and animals. Upon consumption, enzymes like salivary amylases break down the starch into smaller molecules such as maltose and glucose. These smaller molecules are then absorbed by cells.
Starch, a carbohydrate, is composed of glucose monomers connected by α 1-4 or α 1-6 glycosidic bonds. The numbers 1-4 and 1-6 indicate the carbon number of the two residues that bond together. Amylose is a type of starch with unbranched chains of glucose monomers, joined solely by α 1-4 linkages. On the other hand, amylopectin is a branched polysaccharide with α 1-6 linkages at the branch points.

Figure 6. Amylose and amylopectin are two distinct forms of starch. Amylose is made up of straight chains of glucose units that are connected by α 1,4 glycosidic linkages. On the other hand, amylopectin consists of branched chains of glucose units that are connected by both α 1,4 and α 1,6 glycosidic linkages. The arrangement of these glucose subunits gives rise to a helical structure in the glucose chains. Although not shown in the data, glycogen shares a similar structure with amylopectin but is even more extensively branched.
Glycogen serves as the storage form of glucose in humans and other vertebrates. It is composed of glucose monomers and acts as the animal equivalent of starch. Typically, glycogen is stored in liver and muscle cells and is a highly branched molecule. When blood glucose levels decline, glycogen is broken down through a process called glycogenolysis to release glucose.
Cellulose, the most abundant natural biopolymer, forms the major component of the cell wall in plants, providing crucial structural support. Wood and paper are predominantly composed of cellulose. It is composed of glucose monomers that are connected through β 1-4 glycosidic bonds.

Figure 7. Cellulose is a type of polysaccharide where glucose monomers are connected in straight chains through β 1-4 glycosidic linkages. This arrangement causes each glucose monomer to be flipped in relation to the next one, resulting in a long, fibrous structure.
As shown in Figure 7, Cellulose, a compound found in plant cells, possesses rigidity and high tensile strength due to the tightly packed and extended long chains of glucose monomers. While human digestive enzymes cannot break down the β 1-4 linkage in cellulose, herbivores like cows, koalas, buffalos, and horses have specialized bacteria and protists in their digestive system, particularly in the rumen and appendix, that produce the enzyme cellulase. This cellulase enables these animals to digest cellulose-rich plant material and convert it into glucose monomers for energy. Termites also possess organisms in their bodies that secrete cellulases, allowing them to break down cellulose as well.
Insects have a tough outer exoskeleton composed of chitin, a type of polysaccharide. Carbohydrates play different roles in various animals. Arthropods, such as insects and crustaceans, possess an external skeleton called the exoskeleton. This exoskeleton safeguards their internal body parts, as depicted in Figure 8 with the example of a bee. The exoskeleton is primarily comprised of chitin, a biological macromolecule that contains nitrogen. It consists of repeating units of N-acetyl-β-d-glucosamine, which is a modified sugar. Additionally, chitin is a vital component of fungal cell walls. Fungi, belonging to the domain Eukarya, form their own kingdom and are distinct from animals and plants.
Conclusion
In conclusion, carbohydrates play crucial roles in providing energy and structural support in living organisms. They encompass monosaccharides, disaccharides, and polysaccharides, each with their unique functions and characteristics. Monosaccharides such as glucose, galactose, and fructose serve as the building blocks of carbohydrates. Disaccharides like lactose, maltose, and sucrose are formed by the combination of two monosaccharide units. Polysaccharides such as starch and glycogen store glucose for future energy needs. The structural polysaccharide cellulose provides strength to plant cell walls, while amylopectin exhibits a highly branched structure. The storage of glucose in the form of polymers ensures its availability while preventing leakage and excessive water uptake. Understanding the structure and function of carbohydrates is essential for comprehending their significance in biological processes and maintaining a balanced diet.